#GMP-A018. Recombinant anti-Human CD3 mAb
A. Description
- Recombinant Anti-Human CD3 mAb is a T cell-activated antibody produced based on OKT3 through humanization modification and genetic engineering. On the premise of maintaining the activation ability of OKT3 monoclonal antibody on T cells, the product can eliminate the immunogenicity of murine antibodies to the greatest extent. The product recognizes the human TCR ε chain to activate the T cell activation signaling pathway, thus stimulating T cell activation and proliferation.
- Recombinant Anti-Human CD3 mAb is expressed by mammalian cells, and is produced with raw materials of pharmaceutical applicable level. The host protein residue, nucleic acid residue and common pathogens are strictly controlled, and the production and quality management procedures of the product comply with GMP regulations to ensure the traceability of the production process and all raw materials.
![]() B. Usage
B. Usage
- In-vitro cultivation of various types of cell.
- Recombinant Anti-Human CD3 mAb can satisfy most T cell expansion requirements and exhibit the same effects as OKT3. For difficult-to-expand cells, the product can be used in combination with Recombinant Anti-Human CD28 mAb (Cat. No: GMP-A063) or NovoNectin® GMP Grade (Cat. No.: GMP-CH38).
C. Quality Control
| Item | Standard |
| Appearance | Transparent liquid |
| Visible particle | Conform to ChP |
| pH value | 6.5–7.5 |
| Purity | ≥ 95% |
| Bioactivity | ≤ 100 ng/mL |
| Endotoxin | < 10 EU/mg |
| Host protein | ≤ 0.005% |
| Exogenous DNA residue | ≤ 100 pg/mg |
| Mycoplasma test | Negative |
| Sterility test | Conform to ChP |
D. Storage
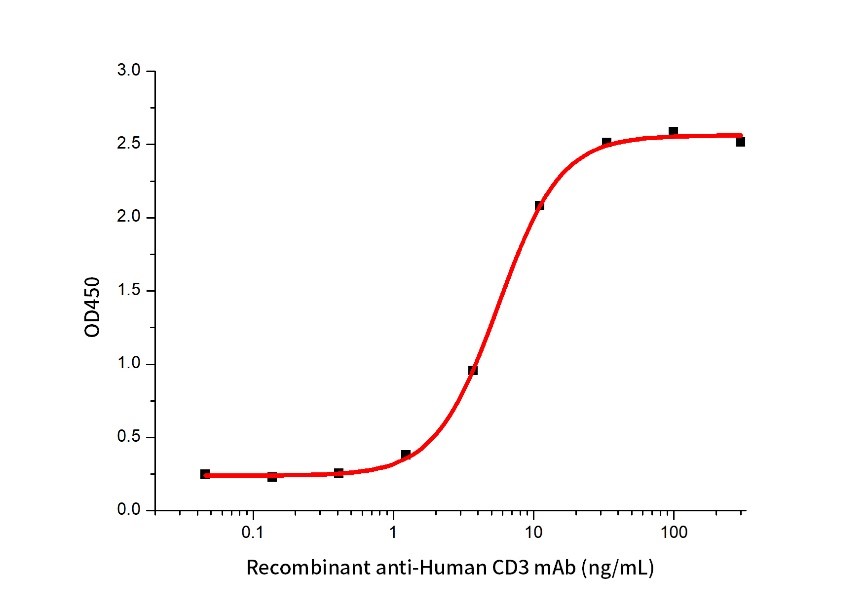
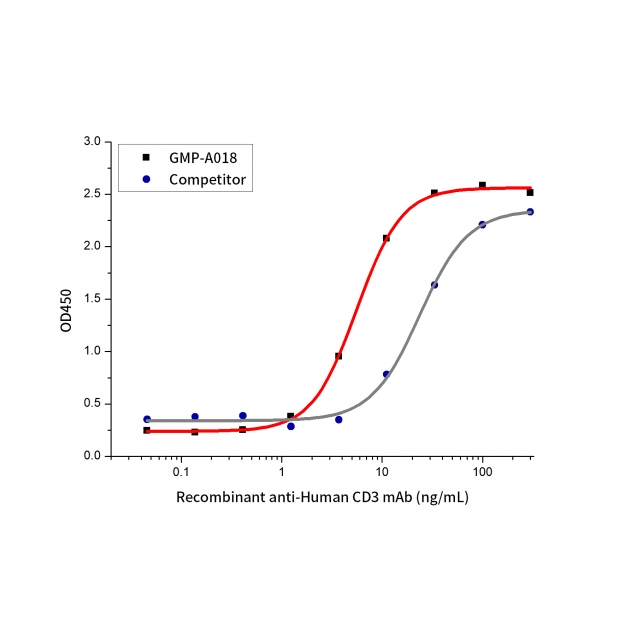
Measured by its ability to induce IL-2 secretion by Jurkat cells. The ED50 for this effect is≤ 100 ng/mL (QC varified).
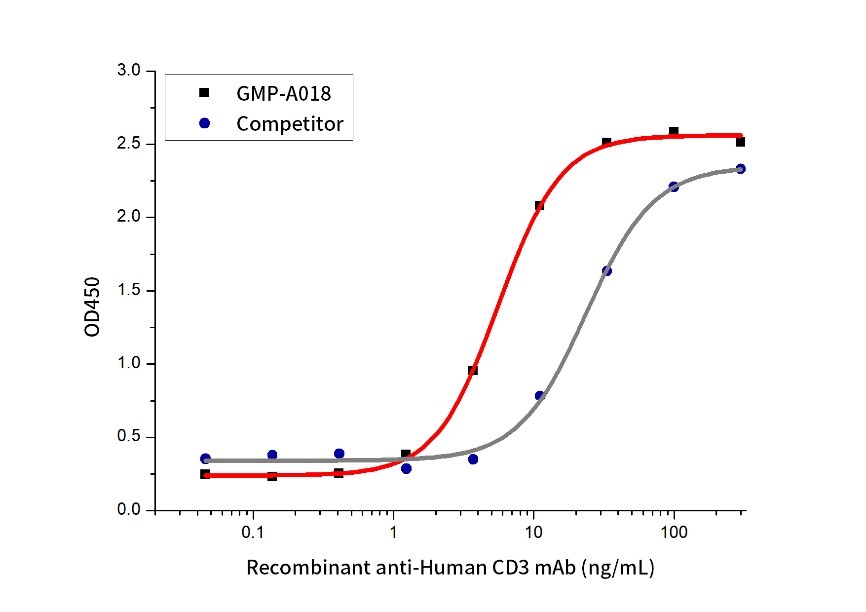
The activity of GMP Human anti-Human CD3 mAb(Cat. No.:GMP-A018) was compared to another commercially available product. Measured by its ability to induce IL-2 secretion by Jurkat cells.
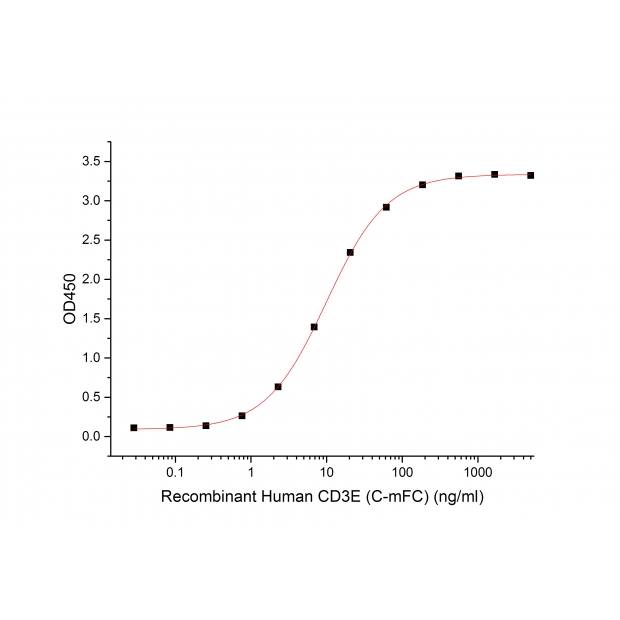
G. Bioactivity-ELISA
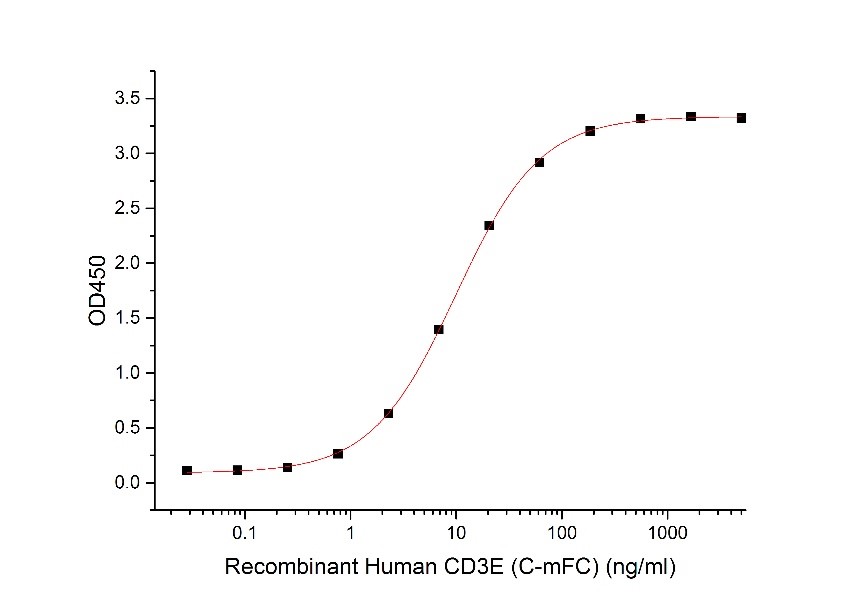
Immobilized Human CD3E-mFc (C00E) at 10μg/ml (100 μl/well) can bind Human Anti-CD3 mAb (GMP-A018). The ED50 of Recombinant Human Anti-CD3 mAb (GMP-A018) is 9.4 ng/ml. (Regularly tested)
For Research or Manufacturing Use Only, Not for Use in Diagnostic or Therapeutic Procedures.
Storage at -20±5℃, expiration date labeled on bottle.
E. Recommendations
- (1)Make sure the product properly stored and none expired before using.
- (2)Sterile condition without pyrogen is required for reconstitution, re-aliquoting, dilution.
- (3)After reconstitution, aliquoted solution vials should be stored at -70°C and minimize freeze-thaw cycles preventing denaturation of protein.
- Applications in T cell activation and expansion:
- General applications: The recommended concentration is 5–10 μg/mL; if necessary, the optimal concentration for specific cells can be selected at 5–20 μg/mL. Coating of culture flask:
- a.Calculate the volume on the basis of 150 μL/cm2, dilute the Recombinant Anti-Human CD3 mAb, recombinant anti-CD28 mAb, and NovoNectin® GMP Grade with normal saline to 5–10 μg/mL, 5–10 μg/mL, and 25–50 μg/mL, respectively, and mix them to a coating solution;
- b.Add the diluted coating solution to a culture flask or bag and cover the bottom with the solution;
- c.Place the solution in a 37 °C incubator for 5–8 h, or at 2–5 °C overnight;
- d.Remove the liquid and wash the flask or bag 3 times with normal saline;
- e.The well-coated culture vessel shall be used immediately; if the vessel is to be used after more than 30 minutes, store it in normal saline.

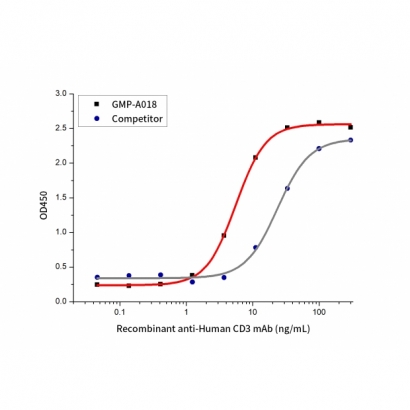
Measured by its ability to induce IL-2 secretion by Jurkat cells. The ED50 for this effect is≤ 100 ng/mL (QC varified).

The activity of GMP Human anti-Human CD3 mAb(Cat. No.:GMP-A018) was compared to another commercially available product. Measured by its ability to induce IL-2 secretion by Jurkat cells.
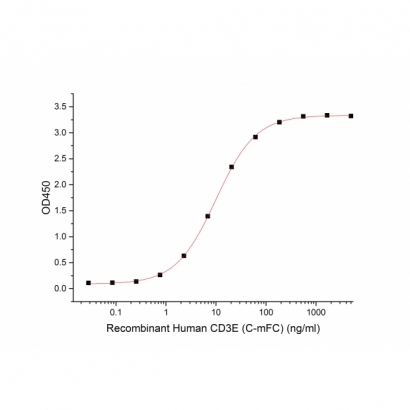
G. Bioactivity-ELISA

Immobilized Human CD3E-mFc (C00E) at 10μg/ml (100 μl/well) can bind Human Anti-CD3 mAb (GMP-A018). The ED50 of Recombinant Human Anti-CD3 mAb (GMP-A018) is 9.4 ng/ml. (Regularly tested)
For Research or Manufacturing Use Only, Not for Use in Diagnostic or Therapeutic Procedures.