SITC 2024: GRIT Biotechnology will Showcase Research Results Utilizing Cas12b Technology for The Preparation of Next-Generation Gene-Edited TIL
From November 6 to 10, 2024, the 39th Annual Meeting & Pre-Conference Programs for Society for Immunotherapy of Cancer (SITC 2024) will be held in Houston, Texas, USA. GRIT Biotechnology will participate in this conference to share research results on next-generation gene-edited TIL supported by the Shanghai Vitalgen BioPharma Co., Ltd. (Vitalgen) AaCas12bMax technology platform. Novoprotein Scientific Inc. (Novoprotein) provided AaCas12bMax enzymes for this research.

The following content is adapted from an article by GRIT Biotechnology.
-----------------------------------------------------------------------------------------------------------------
Title: Development of a next-generation dual-KO TIL product GT300 with AaCas12bMax nuclease.
Presentation No.: 464
Presentation Dates (Local Time): Thursday, Nov. 9
Location: Exhibit Halls A B, George R. Brown Convention Center.
TILs (tumor-infiltrating lymphocytes) have demonstrated promising therapeutic effects in various solid tumors, including melanoma, significantly extending the survival of advanced-stage patients. However, the efficacy of TILs in certain "cold tumors" with poor lymphocyte infiltration, the efficacy of TIL still needs to be evaluated and improved. Developing the next-generation of gene-edited TIL therapies will be an ideal strategy to improve TIL function.
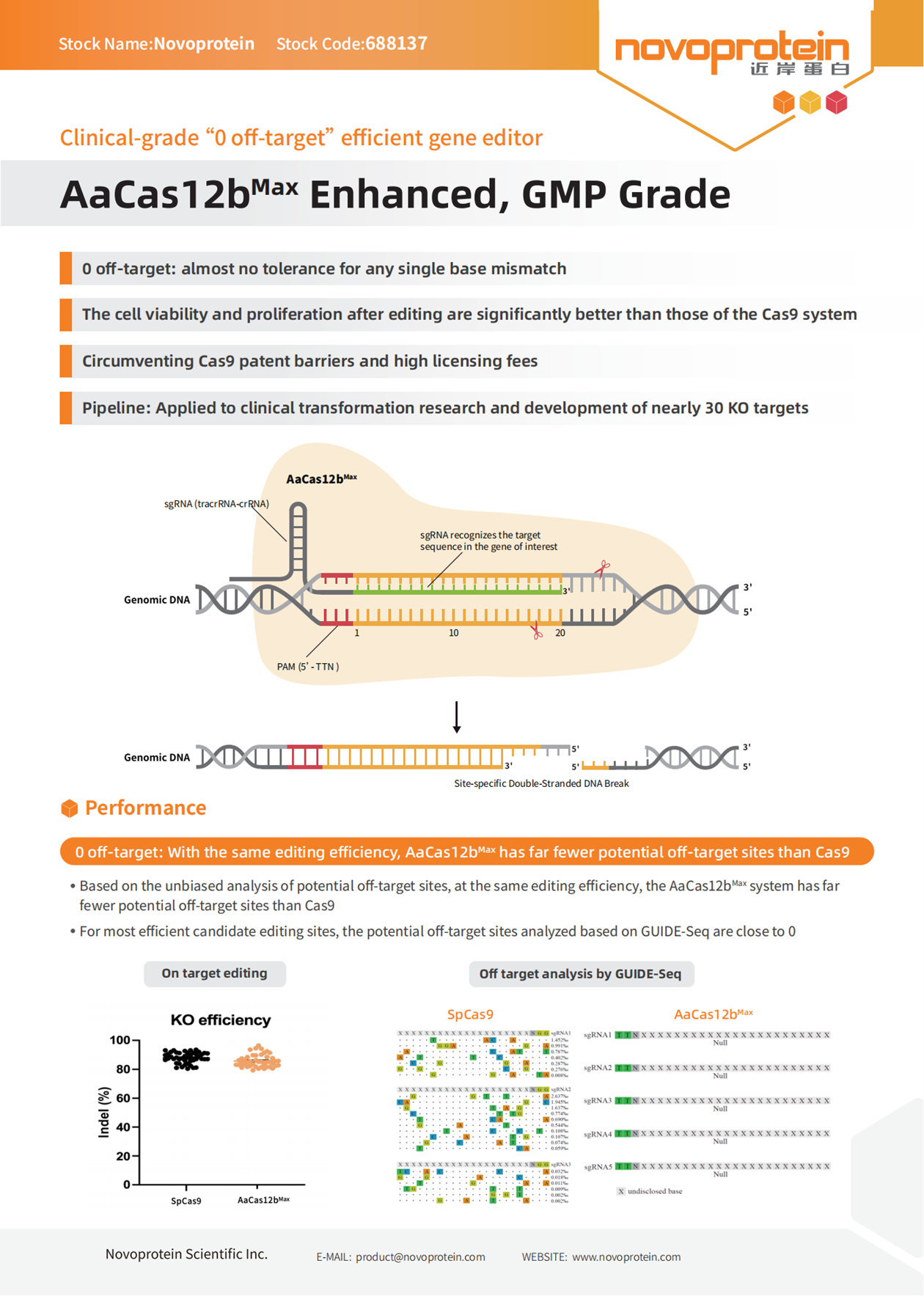
Cas12b, an effector nuclease in the CRISPR family, surpasses the commonly used SpCas9 nuclease in editing precision while maintaining high editing efficiency. This makes it an ideal choice for gene editing applications, effectively reducing off-target toxicity. In this study, GRIT Biotechnology applied the Vitalgen AaCas12bMax technology to develop a next-generation dual-KO tumor-infiltrating lymphocyte (TIL) product—GT300.
In this project, computer-assisted prediction technology was employed during the design of sgRNAs for AaCas12bMax to minimize the risk of mismatches across the whole genome. Subsequently, more optimal sgRNAs were screened in HEK293 cells and human PBMC T cells for further evaluation of their editing efficiency and effectiveness in TIL products.
The results showed that the screened sgRNAs used in the AaCas12bMax system achieved gene knockout efficiencies comparable to the traditional SpCas9 system in TIL cells. Additionally, in a mouse PDX model, TIL with gene knockouts using AaCas12bMax demonstrated comparable performance to those edited with the traditional SpCas9 system, exhibiting good cell killing ability, in vitro cytokine release ability, and tumor suppression effects.
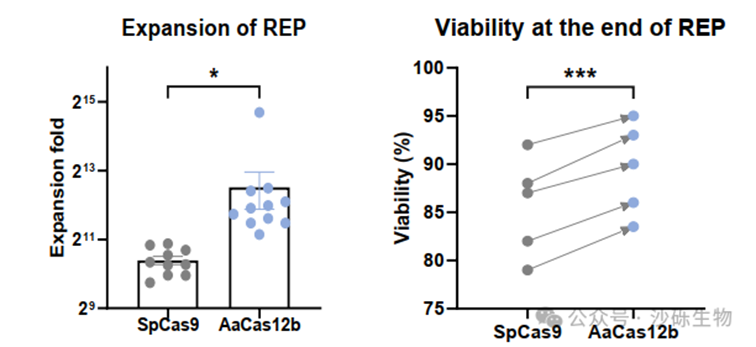
Fig 1. Enhanced Expansion Capability of AaCas12bMax-Edited TIL Products.
Notably, TIL products edited with AaCas12bMax demonstrated significantly improved cell expansion ability and cell survival ability, with a total cell count increase of 3 to 5 times following TIL expansion. Furthermore, these AaCas12bMax-edited TIL products exhibited higher stemness, lower exhaustion characteristics and apoptosis rates, as well as enhanced expansion capacity after in vivo reinfusion (Fig 1).
This indicates that, compared to the traditional SpCas9 system, gene editing with AaCas12bMax causes less stress damage to TIL products, resulting in better cell status for the edited TIL.
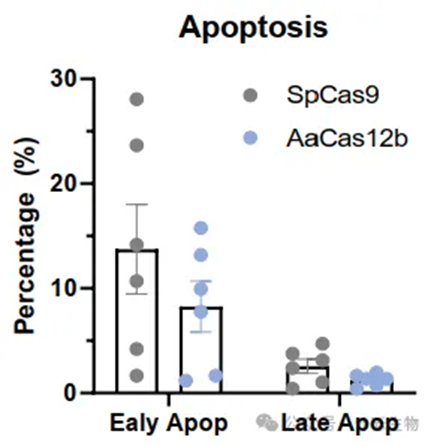
Fig 2. Lower Apoptosis Levels in AaCas12bMax-Edited TIL Products.
RNA-seq studies further revealed that T cell products edited with AaCas12bMax exhibited faster DNA repair speed, along with lower apoptosis compared to SpCas9 (Fig 2). Additionally, AaCas12bMax shows improvements in cellular state impairments related to pyroptosis and abnormal activation of exhaustion pathways compared to SpCas9 editing.
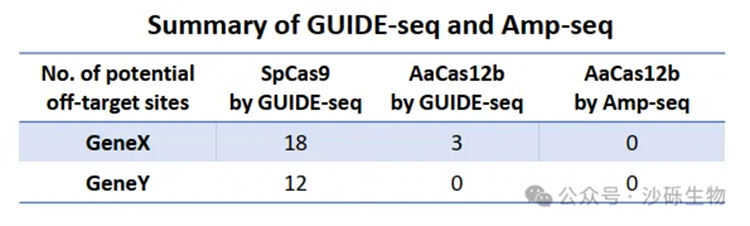
Fig 3. Fewer Off-Target Events Occurring with AaCas12bMax Editing.
At the same time, SITE-seq and GUIDE-seq analyses revealed a significant reduction in off-target sites and a marked decrease in off-target effects in TIL products edited with AaCas12bMax (Fig 3). Additionally, chromosomal translocation analysis using PEM-seq indicated that AaCas12bMax induced fewer chromosomal translocation events.
The results of this study utilizing AaCas12bMax for TIL editing demonstrate that AaCas12bMax achieves high editing efficiency and minimal off-target effects in TIL products, significantly enhancing the expansion capability, cell viability, and in vivo adaptability after reinfusion.
We extend our special thanks to Novoprotein for developing and optimizing the AaCas12bMax process and for providing AaCas12bMax enzymes for this research.
GRIT Biotechnology has applied the Vitalgen AaCas12bMax technology to develop the next-generation dual-KO TIL product—GT300. Currently, clinical research for this product is underway, enrolling patients with various types of advanced unresectable or metastatic solid tumors. The recruitment, enrollment, and clinical research activities are ongoing at multiple clinical centers.
-----------------------------------------------------------------------------------------------------------------
Novoprotein offers GMP-grade and RUO-grade AaCas12bMax based on a mature, large-scale GMP production platform. Additionally, to facilitate customers in obtaining test results quickly, Novoprotein can also provide universal target-specific sgRNAs or sgRNA sequences.
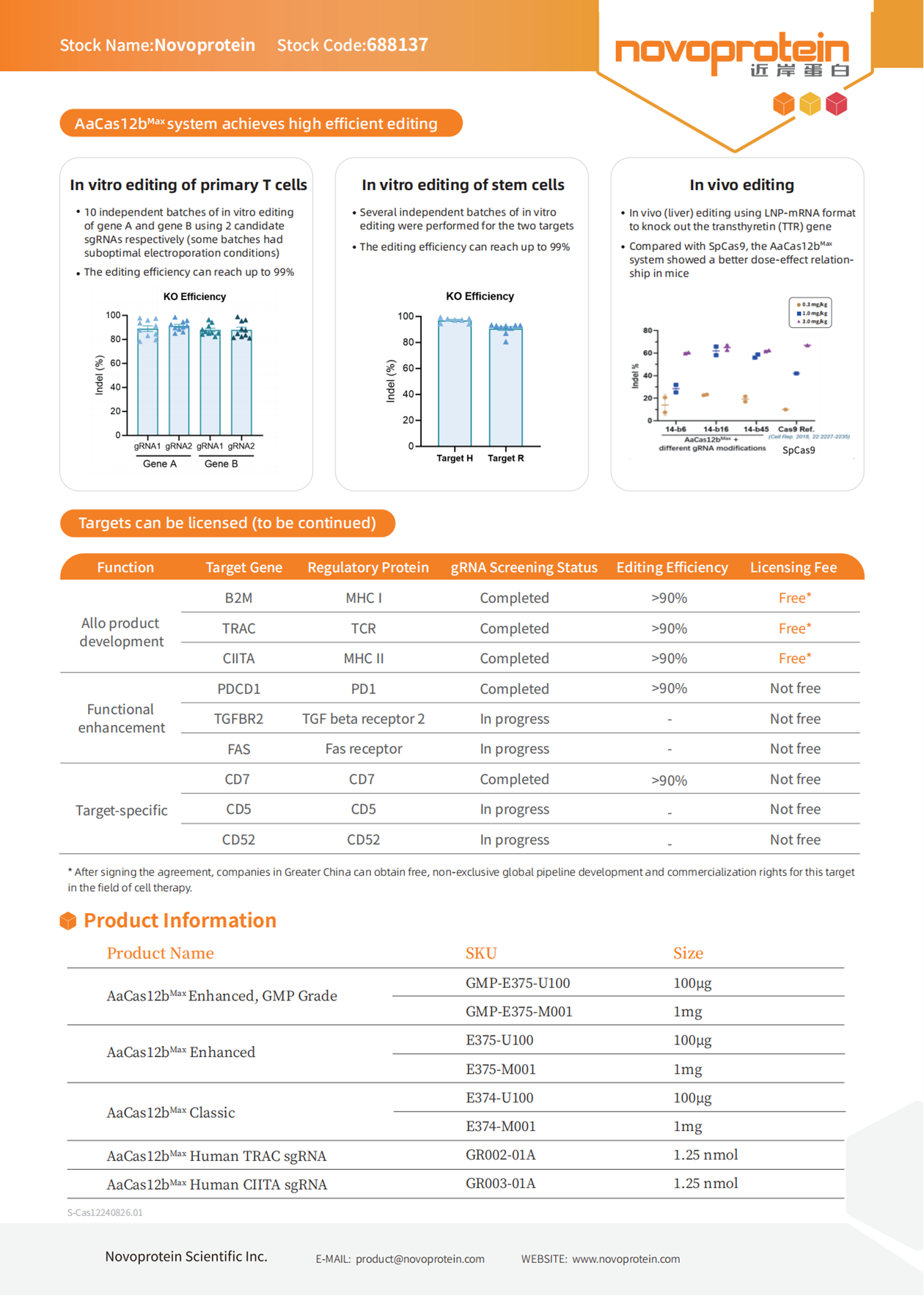
.png)
| Cat# | Product Name | Application |
| GMP-E375 | AaCas12bMax Enhanced,GMP Grade | 100μg, 1mg |
| E375 | AaCas12bMax Enhanced | 100μg, 1mg |
| GR002 | AaCas12bMax Human TRAC sgRNA | 1.25nmol |
| GR003 | AaCas12bMax Human CIITA sgRNA | 1.25nmol |
| E131 | T7 High Yield RNA Transcription kit | 50rxns, 100rxns |
| N243 | RNA Clean Beads | 5ml, 40ml |
| E035 | 2×Fast Pfu Master Mix | 1ml, 1ml×5 |
| M017 | T7 Endonuclease I | 250U, 250U×4 |
| N240 | NovoNGS® DNA Clean Beads | 5ml, 60ml, 450ml |
